Previous research has shown a correlation between blood sugar or glycemic load and cancer growth for a number of types of cancer. Those studies were retrospective and/or studies of fewer than 20 human subjects and/or studies on mice. This study is a 7-year interventional study of 317 human patients at one clinic, who were treated naturopathically, with anti-neoplastic nutrients and herbs, plus the recommended dietary intervention of abstention from sweetened foods.
Methods
We analyzed the clinical significance (mortality) of sweetened food consumption among cancer patients at our clinic. Since 2006, this clinic has collected data on sugar consumption in cancer patients, and has actively recommended, but never enforced in any way, avoiding the consumption of sweetened foods (except with the sweetener Stevia Rebaudiana). In this controlled interventional study, we followed the diets and outcomes of all 317 cancer patients who came to our clinic with a diagnosis of cancer, and who stayed at least two weeks in our care. All results are reported in this paper.
Results
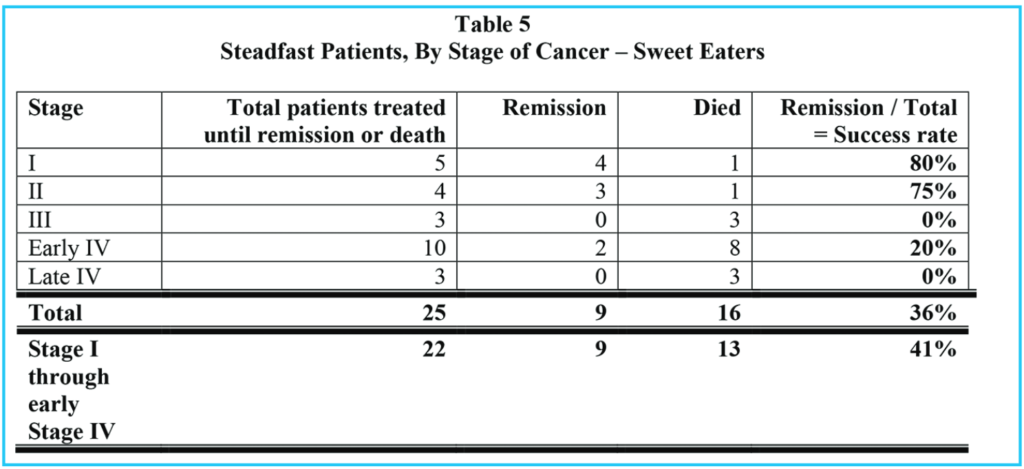
The remission rate is significantly different for the following two categories: all patients: 151 / 317 = 48% and those who ate sweetened foods: 9 / 29 = 31%. However, the difference in these two groups is much more pronounced if we consider those patients who continued our treatments until either remission or death. Comparing all patients who were steadfast in our recommended naturopathic treatments with the sweetened food eaters who were steadfast in all but dietary recommendations, 151 / 183 = 83% of all totally steadfast patients went into remission, but only 9 / 25 = 36% of the steadfast sweetened food eaters went into remission.
Of all patients who were steadfast in the treatments (including our sweetened food eaters), 32 / 183 = 17% died while still under our care, but considering only the sweetened food eaters who were otherwise steadfast in the treatments, 16 / 25 = 64% died.
Conclusion
Consuming sweetened foods (other than stevia sweetened foods) made a significant difference in patient outcome across all stages and all types of cancer. We therefore recommend that the diet of cancer patients not contain sweetened foods other than stevia.
Background and Methods
We analyzed the clinical significance (mortality) of sweetened food consumption among cancer patients. Since 2006, this clinic has collected data on sugar consumption in cancer patients, and has actively recommended, but never enforced in any way, abstention from the consumption of sweetened foods. This clinic has no inpatient facilities and no food service. All patients chose all of their own food, all of which originated from outside this clinic. Data from all 317 patients who came to us with a diagnosis of cancer are included in this interventional study, excluding only those cancer patients who decided against further treatment after less than two weeks in our care. We treated with natural methods alone, choosing among methods with research established anti-neoplastic effect, both oral and intravenous, dietary and supplemented, nutritional and herbal, having a preference for those with high patient tolerance and compatibility, and varying with individual needs and tolerance, according to the standard naturopathic principle of “Treat the whole person.”
Dietary interventions are of the utmost importance in cancer therapy, especially keeping blood sugar low. The significant majority of research on the subject establishes a correlation between blood glucose and tumor growth. Using PET imaging preferentially for tumor evaluation, clinicians make use of the fact that tumors take up blood glucose disproportionately over benign tissue, which implies an especially glucose dependent metabolism in cancer cells. In fact, the difference between up-take of glucose in a malignant tumor and uptake in normal tissue is so stark that the rough outlines of a tumor may be seen on a PET simply from the borders of where glucose uptake is strong next to where it is weak.
Research has shown a correlation between blood sugar or glycemic load and cancer growth for pancreatic cancer,1,2,3,4 breast cancer, 5,6,7,8 prostate cancer,9,10gastric cancer, 11,12 colorectal cancer, 13,14,15,16 ovarian cancer, 17,18 endometrial cancer,19,20and liver and biliary tract cancers.21,22 Given all of this evidence, it would be reckless for a physician to allow a cancer patient to assume that sugar intake is harmless. We therefore ask all of our cancer patients to avoid sweeteners, such as sugar, honey, maple syrup, corn syrup, high fructose corn syrup, alcohol, alcohol sugars and plant nectars, as well as fruit juices, because such foods tend to have the highest glycemic indices. Use of stevia is encouraged if and when a sweetener is desired. For the same reason, we asked patients to also limit other refined carbohydrates, specifically flour products. Whole natural foods: vegetables, fruits, whole grains, eggs, dairy and other animal proteins are encouraged as the entire diet, with the widest available variety in those groups. Many patients arrive to our clinic already consuming all of those types of foods. Others arrive with different diets. Some patients have chosen a vegan diet. Others have chosen an ovo-lacto-vegetarian diet. Many others are omnivores. Others avoid grains altogether. We have not actively pushed our patients to one or the other of these diets, because we tried to maintain the primary dietary focus on the avoidance of sweeteners, without distraction by other dietary priorities. Keeping the focus exclusively on the avoidance of sweeteners seems to minimize the opportunity to forget that one guideline. Patients may eat absolutely anything they like, except that we strongly urge the avoidance of sweeteners, except for Stevia rebaudiana, which has no significant sugar content. Through repeated reminding, with gentle, encouraging consultation and troubleshooting of sugar cravings, as well as brainstorming of alternative foods that may satisfy those cravings, during patient consults, we create a situation where our patients are unlikely to completely forget our recommendation when given a choice of whether to have dessert or skip dessert.
 The overwhelming influence of the oncology profession on diet has suppressed this type of recommendation among many physicians. Chemotherapy IV rooms are known for having candy dishes in plain sight. Most oncologists have generally recommended that cancer patients eat desserts so intently that it seems the patients’ primary responsibility is to keep their weight up, without regard for specific health effects of various foods. Under this sugar-oriented food culture, both in the American culture at large and in the oncology clinic, other physicians less specifically credentialed to treat cancer patients shrink from challenging this dictum of the oncologists.
The overwhelming influence of the oncology profession on diet has suppressed this type of recommendation among many physicians. Chemotherapy IV rooms are known for having candy dishes in plain sight. Most oncologists have generally recommended that cancer patients eat desserts so intently that it seems the patients’ primary responsibility is to keep their weight up, without regard for specific health effects of various foods. Under this sugar-oriented food culture, both in the American culture at large and in the oncology clinic, other physicians less specifically credentialed to treat cancer patients shrink from challenging this dictum of the oncologists.
However, when we started this dietary recommendation to our cancer patients in 2006, the time was already more than ripe to rebel against the sweet-tooth trend, because most of the above cited research on sugar consumption and tumor growth had already been published. So at our clinic we decided to acknowledge the little known but already well-established connection between sugar and cancer, and thus to recommend sugar avoidance to our cancer patients.
Sugar and Its Effect on the Body
So let’s look at what we mean by “sugar.” Commonly the word “sugar” means sucrose, derived from sugar cane. Sucrose, a disaccharide, is a compound of glucose and fructose, each a monosaccharide, and sugar is composed equally of both. High-fructose corn syrup is similar, except that it has a higher proportion of fructose to glucose. 80% of sucrose used worldwide is from cane sugar; most of the rest is from beets. It is already common knowledge that sugar is “empty calories,” that is, no protein or fat or complex carbohydrates. In its refined form it contains no nutrients at all, no vitamins, minerals, flavonoids or other antioxidants, no fiber, no amino acids. However, sugar is far more damaging to the health than simply the null effect of empty calories.
Epidemiologically, sugar consumption was thought to be 40 pounds per person per year in the U.S. in 1986.23 By the early 2000s, Americans were consuming 90 pounds per person per year, which coincided with the time that one-third of Americans were obese and 14 million were diabetic..24 The most likely explanation of this correlation is that sudden intake of a large amount of sugar overwhelms the liver, which then turns excess sugar to fat, primarily palmitate – a saturated fatty acid, and puts triglycerides in the bloodstream. This process is also thought to correlate with insulin resistance, as I’ll discuss below.
Population studies have corroborated these findings in various countries, but the idea that sugar could be deleterious to the health fell into disfavor in the 1970s, as American nutritionists at that time followed en masse Ancel Keys and his Seven Countries Study. This study, implicating saturated fat in cardiovascular disease, had actually been a 22-country study, in which those countries that contradicted the hypothesis were quietly dropped from the discussion. Incidentally, those same countries were found to have a direct relationship between sugar consumption and heart disease, but that was not the widely publicized conclusion. Saturated fat became the scapegoat. In the following decades, salt would come to play the role of villain. As country after country fell victim to the damaging effects of sugar in the diet, one scapegoat after another took the blame. The British physiologist John Yudkin found an effect of sugar consumption on obesity, diabetes and cardiovascular disease,25 and brought the public’s attention to the health effects of sucrose in his 1972 book Pure, White and Deadly.26 Yudkin was often personally attacked, quite virulently, for writing about the pathological conditions either caused by or worsened by sugar. In 1975, William Dufty challenged the conventional thinking on sugar with his bestseller Sugar Blues.27 Then Nancy Appleton wrote “141 Reasons Sugar Ruins Your Health,”28 and Lick the Sugar Habit.29 In the last few years, Robert Lustig has explained the widespread dam-age in a way that the public is beginning to appreciate. But the best at breaking down the chemistry to clear language as well as the politics, intrigue and history of American food fights is journalist Gary Taubes, author of “Is Sugar Toxic?”30 as well as the decade-old but still current “What If It’s All Been A Big, Fat Lie?”31 and “Why We Get Fat.” 32
Sugar is broken down in the duodenum by sucrase and isomaltase glycoside hydrolases. A rapid rise in blood glucose quickly follows ingestion of pure sucrose, or sucrose-rich solids and especially liquids. Sweets accompanied by fats, proteins or fiber will enter the bloodstream slower than sweets alone in a refined carbohydrate vehicle, such as a cookie. But whether fast or slow, insulin is secreted by the pancreas in response to the presence of sugar in the blood. A lot of sudden sugar in the blood results in a lot of insulin secreted by the pancreas. When that happens too much or for too many years, the pancreas be-comes depleted and can’t keep up with the body’s demand for insulin. Blood sugar rises beyond normal range, and diabetes is the diagnosis. In animal studies of sugar bingeing this process began in only a few weeks.33 Chronically high insulin has other effects besides diabetes: atherosclerosis and hypertension, and unfavorable HDL/ LDL ratios.
This is where cancer comes in: First, we have to look at epidemiology again. The WHO International Agency for Research on Cancer found that cancer is more prevalent in populations where there is obesity, diabetes and metabolic syndrome.3 The likely cause and effect is that sugar consumption causes insulin secretion, and that insulin, as well as its closely related hormone, insulin-like growth factor, pro-motes tumor growth. One effect of IGF-1 is to deliver sugar into a cell, among other things. However, very high protein diets can also result in elevated IGF-1. It can bind to insulin receptors, and like insulin, the receptor for IGF-1 is a receptor tyrosine kinase. Too much IGF-1 can result in a transient hypoglycemia. IGF-1 acts as a growth factor in breast cancer,35 prostate cancer,36 and lung cancer,37 among other cancers.
Tumor growth is thought to occur by the fact that insulin delivers sugar to cells and that cancer cells are thought to be more dependent on sugar than normal cells. Whereas normal cells down-regulate their receptors after a certain level of saturation with sugar, cancer appears to be insatiable. Cancer’s rapid growth seems to place no limit on the sugar it can use. Insulin delivers that sugar. Some cells develop mutations to enhance insulin’s influence on the cell’s sugar uptake. Craig Thompson MD, President of Memorial Sloan Kettering Cancer Center in New York has studied insulin and IGF’s influence on cancer cells and has said he believes that insulin is what drives malignant tumors to take up more and more blood sugar and to metabolize it, and that it is this process that allows many precancerous cells to undergo the mutations that make them malignant.38
 But what do cancer cells get from sugar that is so useful to their growth? We know that sugar provides quick energy, and that not a lot of processing needs to happen before the body and brain use sugar as fuel. Cancer grows faster than normal tissue, so we can see the expedience of using sugar as a fuel. But unlike normal cells, cancer can live where there is little oxygen. So instead of a normal metabolism, that is cellular respiration, cancer cells preferentially undergo anaerobic fermentation, which converts NADH to NAD+, which then enables anaerobic or aerobic glycolysis. Otto Warburg discovered this difference between normal and malignant cells in 1924.39 Initially, he thought that all cancer cells used only anaerobic glycolysis to produce energy, but it is now known that cancer is capable of both kinds of metabolism. The beginning and end is that cancer cells convert sugar to lactic acid. No oxygen means no electron transport chain. Even in the presence of adequate oxygen levels, cancer cells seem to default to fermentation rather than oxidative phosphorylation to produce ATP, although ATP is formed much more efficiently from the electron transport chain and oxidative phosphorylation than with fermentation. For the large amount of sugar metabolized in fermentation, little ATP is formed. It may be the case that because this fermentation process is so inefficient in its production of ATP that only large amounts of sugar and a high rate of sugar uptake will work to drive rapid tumor growth, and this is likely why cancer is so dependent on the presence of sugar.
But what do cancer cells get from sugar that is so useful to their growth? We know that sugar provides quick energy, and that not a lot of processing needs to happen before the body and brain use sugar as fuel. Cancer grows faster than normal tissue, so we can see the expedience of using sugar as a fuel. But unlike normal cells, cancer can live where there is little oxygen. So instead of a normal metabolism, that is cellular respiration, cancer cells preferentially undergo anaerobic fermentation, which converts NADH to NAD+, which then enables anaerobic or aerobic glycolysis. Otto Warburg discovered this difference between normal and malignant cells in 1924.39 Initially, he thought that all cancer cells used only anaerobic glycolysis to produce energy, but it is now known that cancer is capable of both kinds of metabolism. The beginning and end is that cancer cells convert sugar to lactic acid. No oxygen means no electron transport chain. Even in the presence of adequate oxygen levels, cancer cells seem to default to fermentation rather than oxidative phosphorylation to produce ATP, although ATP is formed much more efficiently from the electron transport chain and oxidative phosphorylation than with fermentation. For the large amount of sugar metabolized in fermentation, little ATP is formed. It may be the case that because this fermentation process is so inefficient in its production of ATP that only large amounts of sugar and a high rate of sugar uptake will work to drive rapid tumor growth, and this is likely why cancer is so dependent on the presence of sugar.
 Does this mean that starving the cancer cell of sugar kills the cancer cell? It is true that some cancer patients, especially those with high grade brain tumors as well as some other types, who have followed a ketogenic diet, which is an extremely low carbohydrate diet, have fared well. To summarize the classic ketogenic diet, fat outweighs the total of protein and carbohydrates 4 to 1 by weight, and total carbohydrates is limited to 20 to 40 grams per day. The classic ketogenic diet eliminates simple and complex carbohydrates: sweeteners, fruits, grains, and starchy vegetables. A later development adds medium-chain triglycerides, such as coconut oil, and a little more variety in the proteins and carbohydrates than the classic ketogenic diet. The lack of carbohydrates in this diet makes metabolism default to burning fats for energy. The liver converts fat to fatty acids and ketone bodies, which the brain can use as fuel in the absence of glucose. A study of ketogenic diet in animal models of glioma found various effects that made glioma cells behave more like normal cells.40 On the gross level in animal studies, a ketogenic diet was found either to reduce the tumor size or slow tumor growth in glioblastoma,41 prostate cancer,42 gastric cancer,43 and lung cancer.44 It was also found to improve quality of life in patients with advanced metastatic disease in a variety of cancers.45 The ketogenic diet is difficult for patients who are underweight, as it is very difficult not to lose more weight with the diet. It is, however, ideal for patients who are overweight, and/or with type II diabetes, as sugar carvings rapidly disappear once patients are in even a mild ketogenic state.
Does this mean that starving the cancer cell of sugar kills the cancer cell? It is true that some cancer patients, especially those with high grade brain tumors as well as some other types, who have followed a ketogenic diet, which is an extremely low carbohydrate diet, have fared well. To summarize the classic ketogenic diet, fat outweighs the total of protein and carbohydrates 4 to 1 by weight, and total carbohydrates is limited to 20 to 40 grams per day. The classic ketogenic diet eliminates simple and complex carbohydrates: sweeteners, fruits, grains, and starchy vegetables. A later development adds medium-chain triglycerides, such as coconut oil, and a little more variety in the proteins and carbohydrates than the classic ketogenic diet. The lack of carbohydrates in this diet makes metabolism default to burning fats for energy. The liver converts fat to fatty acids and ketone bodies, which the brain can use as fuel in the absence of glucose. A study of ketogenic diet in animal models of glioma found various effects that made glioma cells behave more like normal cells.40 On the gross level in animal studies, a ketogenic diet was found either to reduce the tumor size or slow tumor growth in glioblastoma,41 prostate cancer,42 gastric cancer,43 and lung cancer.44 It was also found to improve quality of life in patients with advanced metastatic disease in a variety of cancers.45 The ketogenic diet is difficult for patients who are underweight, as it is very difficult not to lose more weight with the diet. It is, however, ideal for patients who are overweight, and/or with type II diabetes, as sugar carvings rapidly disappear once patients are in even a mild ketogenic state.
From these observations, we may not be able to jump all the way to the conclusion that sugar causes cancer, or even that the elimination of sugar eliminates cancer. However, we can certainly become alert to a cancer patient’s risks in continuing the consumption of sugar, and the possible benefit from eliminating it from the diet.
Results
Regarding the patients at our clinic, 29 patients acknowledged to us that they had disregarded or somehow not adhered to our main dietary recommendation; that is, that they ate sweetened foods at some time during their treatment. The doctors and staff try never to have a judgmental approach to our patients. If a patient has acknowledged that he or she has not ab-stained from sweets entirely, then we take a co-responsible (some might call it co-de-pendent) approach. We take responsibility for not having sufficiently encouraged or offered ideas for adequate and satisfactory substitutes for sweetened foods. So then during one-on-one consults we try to offer more, and more individually applicable, suggestions for the sweet cravings. For example, one person may be more drawn to alcohol, while another is more drawn to chocolate. Yet another may have a coffee habit in which coffee tastes bad to them without sugar. For others, it is ice cream that they want. Whether we were successful or not in persuading such individuals to adopt our recommended diet, we report that category of patient below as one who disregarded our dietary recommendation, unless they agreed to give up sweets at the beginning of treatment and stayed stead-fast in that diet.
Our data is reported as of July 1, 2013. 20 patients died while still exclusively in our care, following all of our protocols, including dietary. These were all types of cancer and all stages of cancer, especially the more advanced stages. 12 more died while still in our care, but having ignored one of our main treatment recommendations, that is to avoid sweetened foods. 16 of our cancer patients have come out of remission. 5 of those are now back in remission. 4 of those 16 had discontinued our main dietary recommendation.
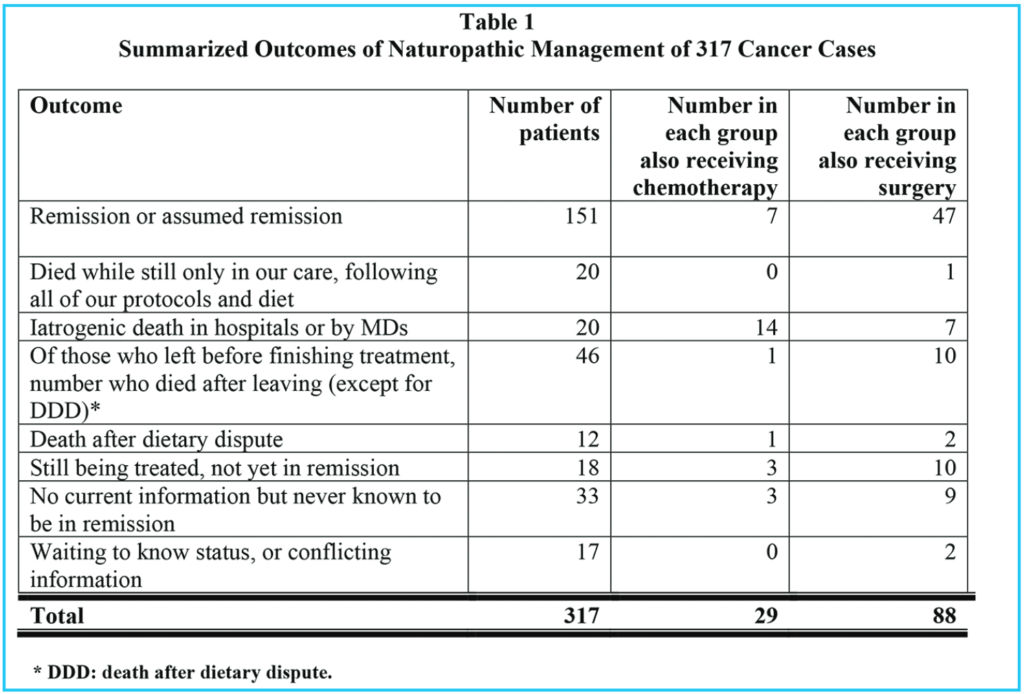
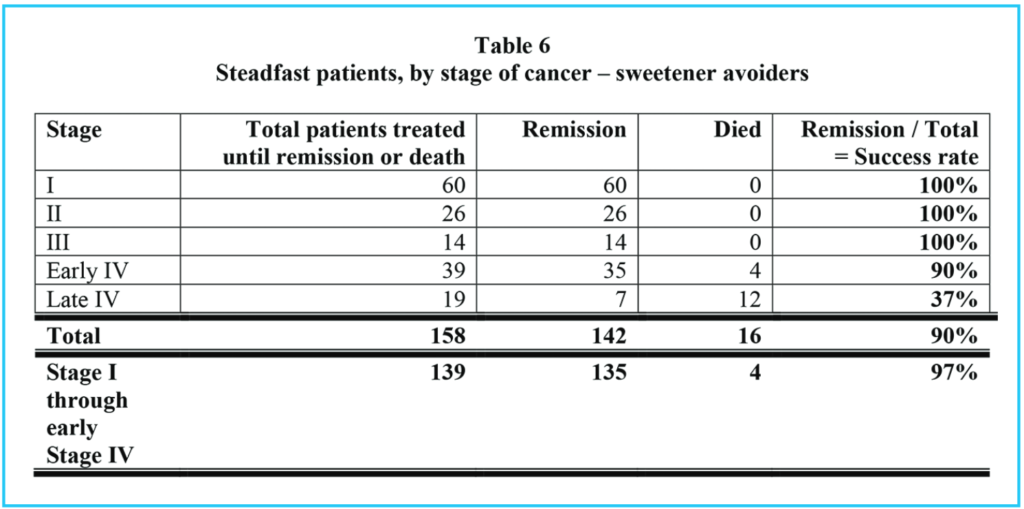
Tables 1, 2 and 3 show comparable information for three groups of patients: Table 1 summarizes all patients who presented to our clinic for cancer treatment, and who stayed in our treatments for at least two weeks. Table 2 shows the same information for those who chose to eat sweetened foods. Table 3 shows the same information for those who chose to avoid sweetened foods. The remission rate is different for all patients: 151 / 317 = 48% and those who ate sweetened foods: 9 / 29 = 31% and those who avoided sweetened foods: 142 / 288 = 49%. However, the difference in these three groups is even more pronounced if we consider those patients who stayed with our treatments until either remission or death, as in Tables 4, 5 and 6.
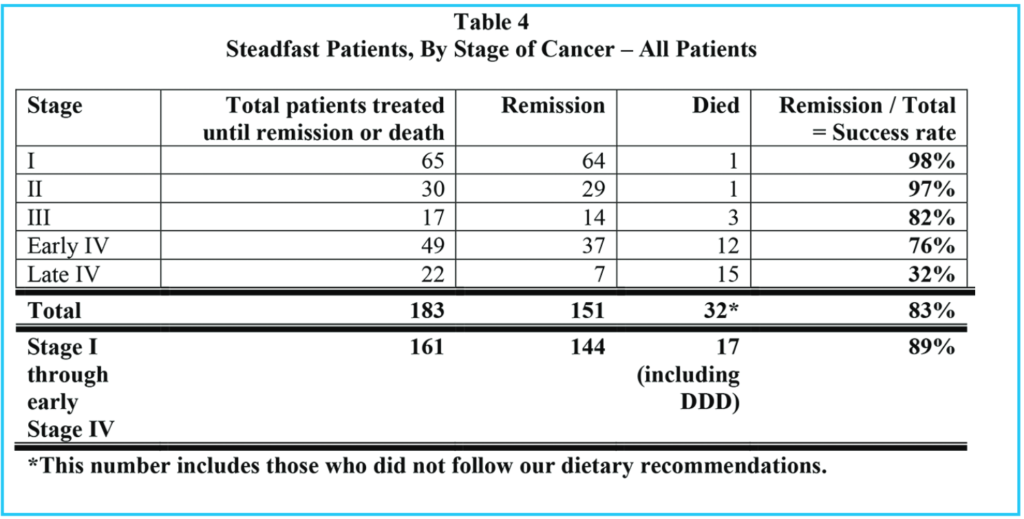
Comparing all patients who were stead-fast in the treatments (Table 4) with the sweetened food eaters, who were steadfast in all but dietary recommendations (Table 5), we see that 151 / 183 = 83% went into remission, but only 9 / 25 = 36% of the sweetened food eaters went into remission. 90% of the steadfast patients who avoided sweeteners went into remission.
Of all patients who were steadfast in the treatments, (including our sweetened food eaters), 32 / 183 = 17% died, but considering only the sweetened food eaters who were otherwise steadfast in the treatments, 16 / 25 = 64% died. Of the steadfast patients who avoided sweeteners, 16 / 158 = 10% died.
Conclusion
Consuming sweetened foods (other than stevia sweetened foods) made a significant difference in patient outcome across both all stages and all types of cancer among patients presenting to our clinic. We therefore recommend that the diet of cancer patients not contain sweeteners other than stevia.
References
1. Chan J, Wang F, Holly E. Sweets, sweetened beverages, and risk of pancreatic cancer in a large population based case-control study. Cancer Causes & Control. 2009 Aug; 20(6): 835-46.
2. Rossi M et al. Dietary glycemic index and glycemic load and risk of pancreatic cancer: a case-control study. Ann Epidemiol. 2010 Jun. 20(6): 460-465.
3. Mueller N et al. Soft drink and juice consumption and risk of pancreatic cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2010 Feb. 19(2). 447-455.
4. Larsson S. Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. Am J Clin Nutr. 2006 Nov. 84(5). 1171-1176.
5. Tavani A et al. Consumption of sweet foods and breast cancer risk in Italy. Ann Oncol. 2006 Feb. 17(2). 341-345.
6. Larsson, S, Bergkvist L, Wolk A. Glycemic load, glycemic index and breast cancer risk in a prospective cohort of Swedish women. Int J Cancer. 2009 Jul 1; 125(1): 153-7.
7. Wu A, Yu M, Tseng C. et al. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr. 2009 Apr; 89(4): 1145-54.
8. Bradshaw P et al. Consumption of sweet foods and breast cancer risk: a case-control study of women on Long Island, New York. Cancer Causes Control. 2009 Oct. 20(8). 1509-1515.
9. Freedland S, Aronson, W. Dietary intervention strategies to modulate prostate cancer risk and prog-nosis. Curr Opin Urol. 2009 May; 19(3): 263-7.
10. Drake I et al. Dietary intakes of carbohydrates in relation to prostate cancer risk: a prospective study in the Malmo Diet and Cancer cohort. Am J Clin Nutr. 2013 Dec. 96(6): 1409-18.
11. Ikeda F, Doi Y, Yonemoto K, et al. Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a population-based cohort study. Gastroenterology. 2009 Aprei(4): 1234-41.
12. Bertuccio P, Praud D, Chatenoud L, et al. Dietary glycemic load and gastric cancer risk in Italy. Br J Cancer. 2009 Feb 10; 100(3): 558-61.
13. Wang B, Bobe G, La Pres J, Bourquin L. High sucrose diets promote intestinal epithelial cell proliferation and tumorigenesis in APC mice by increasing insulin and IGF-1 levels. Nutr Cancer. 2009; 61(1): 81-93.
14. Wang B, Bobe G, La Pres, Bourquin L. Dietary carbohy-drate source alters gene expression profile of intestinal epithe-lium in mice. Nutr Cancer. 2009; 61(1): 146-55.
15. Nayak S. A case control study of roles of diet in colorectal carcinoma in a South Indian population. Asian Pac J Cancer Prev. 2009 Oct-Dec. 10(4). 565-568.
16. Williams C. Dietary patterns, food groups, and rectal cancer risk in whites and African-Americans. Cancer Epidemiol Bio-markers Prev. 2009 May. 18(5). 1552-1561.
17. Augustin L, Polesel J, Bosetti C, et al. Dietary glycemic in-dex, glycemic loan and ovarian cancer risk: a case-control study in Italy. Ann Oncol. 2003 Jan; 14(1): 78-84.
18. Silvera S et al. Glycaemic index, glycaemic load and ovar-ian cancer risk: a prospective cohort study. Public Health Nutr. 2007 Octo. 10(10). 1076-1081.
19. King M, et al. Consumption of sugary foods and drinks and risk of endometrial cancer. Cancer Causes Control. 2013 Jul 24(7) 1427-1436.
References for this article continued at http://www.cancerstrategiesjournal.com/ReferencesVolume2Issue2.pdf